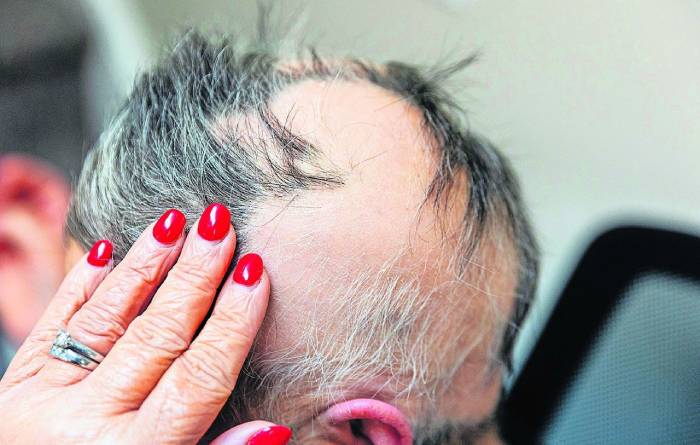
On Monday, the Food and Medicine Administration approved a drug to treat severe alopecia, a condition that affects more than 300,000 Americans each year.
The FDA said in a news release on Tuesday that the approval of Olumiant (baricitinib) was significant since it is the first treatment approved by the government that addresses the autoimmune condition across the body rather than in a single place.
“Access to safe and effective treatment options is crucial for the significant number of Americans affected by severe alopecia,” said Kendall Marcus, M.D., director of the FDA’s Center for Drug Evaluation and Research’s Division of Dermatology and Dentistry.
“Today’s approval will help fulfill a significant unmet need for patients with severe alopecia areata.”
Alopecia patients lose clumps of hair when their immune system attacks their own hair follicles. According to the FDA, Olumiant inhibits the activity of a family of enzymes that cause the attack to flare up.
According to the researchers, more than a third of patients who took 4 mg of the medicine every day raised their scalp hair coverage from 50% to 80% in randomised trials.
According to officials, side effects include upper respiratory infections, headaches, acne, high cholesterol, weight gain, shingles, and urinary tract infection, among others.
According to the press release, “Olumiant is also approved for the treatment of COVID-19 in certain hospitalised adults.”
Will Smith rushed the stage of the Academy Awards earlier this year to assault Chris Rock when the comedian made a joke about his wife Jada Pinkett Smith’s baldness, which is caused by the condition.