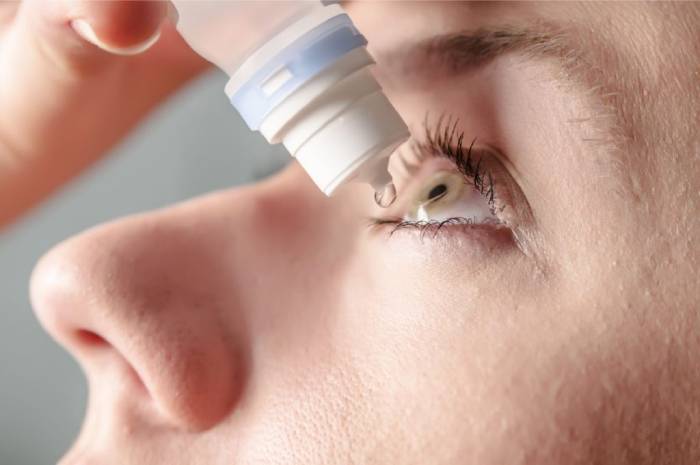
Vuity, an eye drop intended to treat presbyopia, or age-related hazy near vision, was approved by the US Food and Drug Administration in October. When people try to focus on anything close to them, their vision becomes hazy. This problem usually begins after the age of 40.
The eye drops are now accessible by prescription in pharmacies across the country, according to Allergan (which is owned by AbbVie). Allergan claims that their eye drop is the first and only FDA-approved treatment for presbyopia.
The drops operate by reducing pupil size using a formula containing pilocarpine, which has been used in other drugs, including ones that treat the eye, according to Allergan’s Vuity website. The eye drops, according to Allergan, begin working 15 minutes after application, can last up to six hours, and have no effect on distance vision.
According to Allergan’s press statement, 750 people aged 40 to 55 with presbyopia participated in clinical tests prior to the FDA’s approval of Vuity. “A statistically significant proportion of participants” were able to read three or more additional lines on a reading chart when compared to the placebo group. Headache and eye redness were the most common side effects (reported by more than 5% of individuals), although no major side effects were noted. Vuity isn’t a replacement for reading glasses, and it won’t help those with other eye problems like myopia (nearsightedness) or hyperopia (farsightedness), which have symptoms that are comparable to presbyopia but aren’t the same thing.
Vuity will cost $79 for a 30-day supply (2.5 millilitre bottle) for many individuals, according to an AbbVie spokesman, but pricing may vary based on the pharmacy or insurance coverage.
- IPL Icon MS Dhoni Makes Unbelievable Record Against SRH - April 26, 2025
- Cougars Coach Kelvin Sampson Chases 800th Career Victory in NCAA Finals - April 8, 2025
- How to Check IIT GATE 2025 Results Online? Complete Guide - March 19, 2025